January's Case of the Month-2024
A Twist of Events
Patient Information:
Age: ~4 years
Gender: Spayed Female
Species: Canine
Breed: English Bulldog
History:
Patient presented for lethargy, inappetence, and hematuria of a few days duration. Patient was pale upon presentation with firm organomegaly of the cranial abdomen on palpation. CBC showed anemia with a leukocytosis and hypoalbuminemia. Radiographs showed severe organomegaly in the cranial abdomen. A slide autoagglutination test was run and appeared positive. Patient was started on prednisone 30 mg SID and Clavamox 250 mg BID. Urinalysis showed a possible infection as well.
Ultrasound Findings:
Liver: Mildly decreased in size, normal shape, and echogenicity. No focal lesions are appreciated. The gall bladder is moderately distended with normal anechoic and hyperechoic unorganized dependent bile that is not resulting in obstruction. No common bile duct dilation is seen.
Spleen: The spleen measures 2.9 cm in depth and is rounded in shape. There is diffuse hypoechoic echogenicity with hyperechoic linear striations creating a lacy echotexture. There is no appreciable blood flow noted throughout the splenic parenchyma with color flow Doppler. The mesentery surrounding the spleen is moderately hyperechoic.
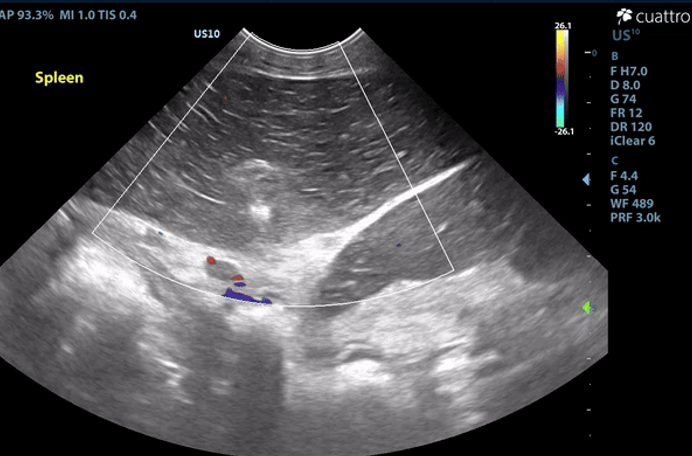
Image 1: Absent color flow doppler signal throughout the splenic parenchyma and vasculature. The spleen is diffusely hypoechoic with hypechoic linear striations producing a lacy echotexture.
Intestinal Tract: The stomach contains a moderate amount of amorphous ingesta as well as amorphous,hyperechoic material associated with a strong anechoic shadow measuring ~7.8 cm in length. The stomach has normal visible wall thickness and layering. The pylorus is free of obstruction. Most loops of small intestine visualized have increased wall thickness measuring up to 6.0 mm with disproportionately large mucosal layers having hyperechoic radiating striations consistent with lymphangiectasia as well as echogenic stippling throughout. The bowel has increased overall motility and liquid contents. No obstruction or masses seen. The colon has normal wall thickness and layering throughout.
Maximum thickness measurements: Stomach- 4.7mm; Duodenum- 2.8mm; Jejunum-6.0mm; Colon- 1.1 mm
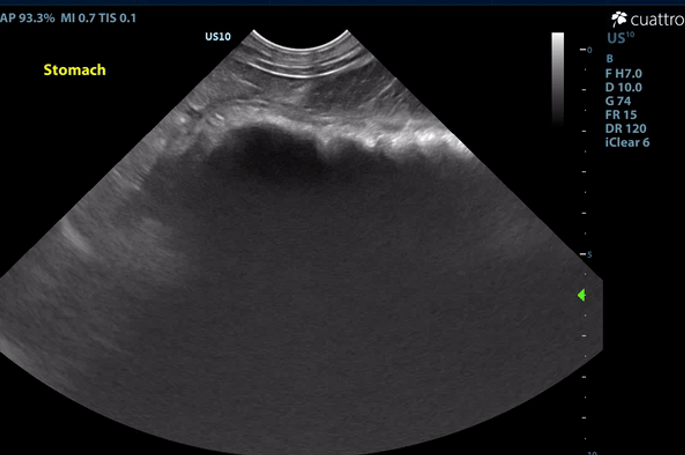
Image 2: Hyperechoic material within the stomach creating a strong anechoic shadow.
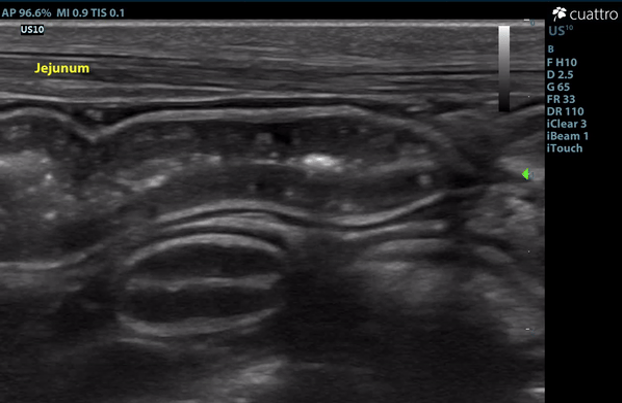
Image 3: Linear striations diffusely throughout the mucosal layer of the jejunum consistent with dilated lacteals.
Serosal Surfaces: A mild amount of anechoic free fluid was noted throughout the abdomen. There is moderately hyperechoic mesenteric echogenicity. No focal lesions noted.
Abdominal Ultrasound Interpretation:
Spleen: The findings are severe. DDX: splenic torsion vs. infarct vs. infiltrative neoplasia vs. open.
Intestines: The findings are moderate. DDx: protein-losing enteropathy (lymphangiectasia), lymphosarcoma, severe inflammatory bowel disease, histoplasmosis, lipogranulomatous lymphangitis). Rule out protein-losing nephropathy (nephrotic syndrome) as a source of protein loss.
Causes of Protein-Losing Enteropathy (PLE)
A. Diseases affecting intestinal lymphatic drainage
- Primary lymphangiectasia (congenital or idiopathic acquired, with breed predisposition in Yorkshire Terrier, Soft-coated Wheaten Terrier, Basenji, Norwegian Lundehund, and Chinese Shar-pei)
- Secondary lymphangiectasia (IBD, Heterobilharzia americana, neoplasia, or congestion secondary to right-sided heart failure or portal hypertension)
B. Acute or chronic inflammatory diseases that result in increased mucosal permeability to protein
- Inflammatory bowel disease (eosinophilic or lymphoplasmacytic enteritis)
- Granulomatous enteritis (histoplasmosis, Pythiosis)
- Intestinal neoplasia (lymphoma, carcinoma)
- Immunoproliferative enteropathy of Basenjis
- Parasitic enteritis in young animals
- Villous atrophy, gluten enteropathy, certain viral and bacterial enteritides
- Chronic obstruction or intussusception
C. Gastrointestinal Blood Loss
- Bleeding tumors
- Ulceration/erosion
- Intestinal parasites (Hookworms)
Gastric Foreign Body: The findings are moderate and suggestive of an intraluminal gastric foreign body vs. recently ingested bone/chew/etc. vs. open.
Microhepatica (decreased hepatic mass): The findings are mild. DDX: Chronic hepatic disease with progressive loss of hepatocytes (cirrhosis or pre-cirrhosis), decreased portal blood flow with hepatocellular atrophy.
- Congenital portosystemic shunt
- Intrahepatic portal vein hypoplasia (presumptive diagnosis - biopsy confirmation is necessary)
- Chronic portal vein thrombosis
- Hypovolemia
- Hypoadrenocorticism
Ascites: This finding is mild. DDx: transudate vs. hemorrhagic vs. exudate.
Mesentery: The findings are moderate. DDx: peritonitis - inflammation vs. paraneoplastic reaction vs. infectious vs. fibrosis vs. other.
Recommendations:
The splenic appearance and lack of color flow Doppler signal is consistent with a splenic torsion or infarct. Exploratory laparotomy is recommended for splenectomy and histopathology.
The ingesta within the stomach is associated with a strong anechoic shadow consistent with foreign material. Exploratory laparotomy is recommended for further evaluation of the intestinal tract and gastrotomy if indicated. The changes to the small intestinal tract are consistent with dilated lacteals, a common finding in dogs with protein-losing enteropathy (PLE). GI biopsies are recommended if surgery is elected.
A red blood cell transfusion is recommended prior to surgery due to the degree of anemia (HCT 15%). Additional diagnostics/therapeutics should be performed as clinically indicated.
Outcome/Further Testing:
Unfortunately, the owner elected for humane euthanasia due to financial constraints.
Necropsy: Post-mortem abdominal exploratory confirmed a splenic torsion, serous ascites, as well as cloth material within the stomach.

Image 4: Gastric foreign material.
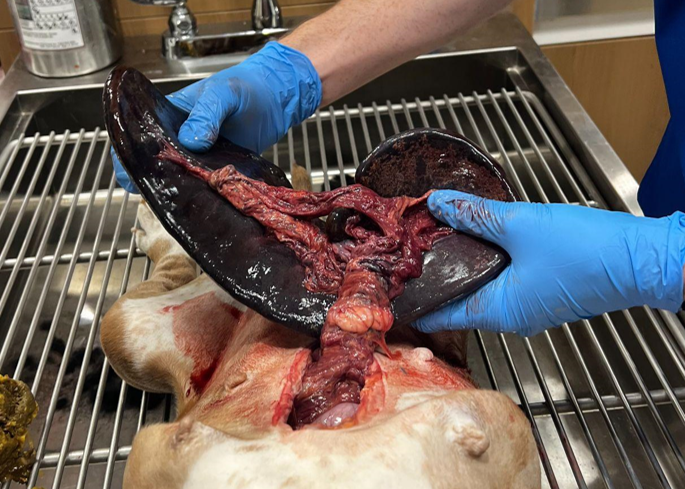
Image 5: Splenic torsion.
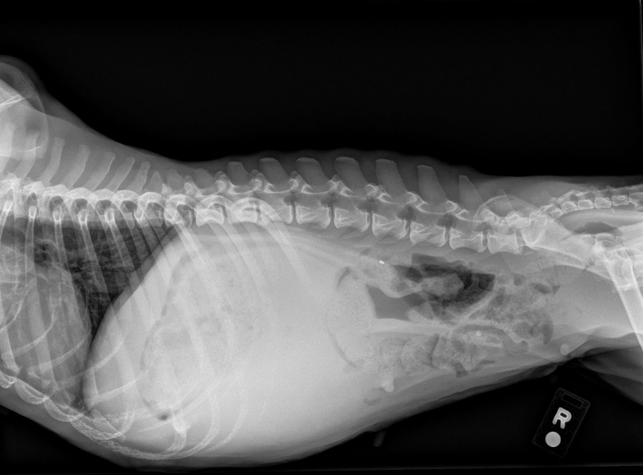
Image 6: Right lateral radiograph.
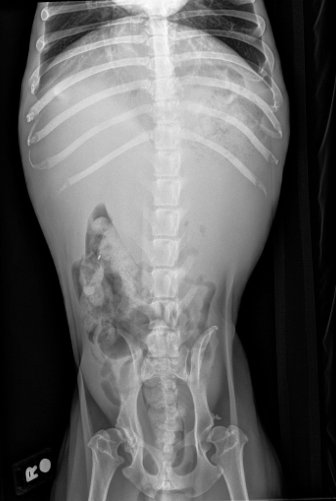
Image 7: VD radiograph.
Discussion:
Multiple pathologies were discovered in this patient, most notably a gastric foreign body, primary splenic torsion (PST), and dilated lacteals consistent with protein-losing enteropathy. Notable clinical pathologies established prior to the ultrasound study included marked anemia (15.9%), moderate leukocytosis (20.2 K), and severe hypoalbuminemia (1.5 g/dL). A positive saline agglutination test (SAT) was also noted. Bacteriuria (rods and cocci), hemoglobinuria, and mild pyuria (3 WBC/hpf) were also noted via SediVue Analyzer, but manual cytology was not reported.
The interconnectivity of these pathologies will be discussed further here. There are three broad categorical causes of anemia:
- Decreased production (e.g., anemia of chronic disease such as erythropoietin-related conditions, iron deficiencies, or bone marrow conditions)
- Loss (hemorrhage)
- Destruction (e.g., hemolysis, oxidative injury, parasitic causes, toxicity)
Further investigation would be necessary to fully elucidate the true etiology/etiologies. However, reasonable assumptions may be made to better understand the anemia. The initial concern for this patient’s anemia was presumed to be an immune-mediated disease (IMHA) due to the degree of anemia, positive saline agglutination test (SAT), and presence of hemoglobinuria.
The mechanism of hemoglobinuria in PST dogs is suspected to be secondary to intravascular or intrasplenic (extravascular) hemolysis. Based on the ACVIM consensus statement on the diagnosis of immune-mediated hemolytic anemia, this patient would be classified as "supportive of IMHA, provided another cause of anemia cannot be identified." However, a positive SAT can result from RBC rouleaux formation, which can be promoted in hypoalbuminemic patients. Further dilution and cytology would be necessary to differentiate between true agglutination and rouleaux formation in light of a positive SAT.
A CBC with pathology review would also be beneficial in this case to assess for regenerative changes, red blood cell morphologies, red blood cell pathogens, etc., to further strengthen the argument for a hemolytic cause of anemia. Given the exhaustive list of reported inciting causes of IMHA, this patient coincidentally (or not) has multiple morbidities associated with triggering IMHA (e.g., inflammatory disease, necrosis, urinary tract infection).
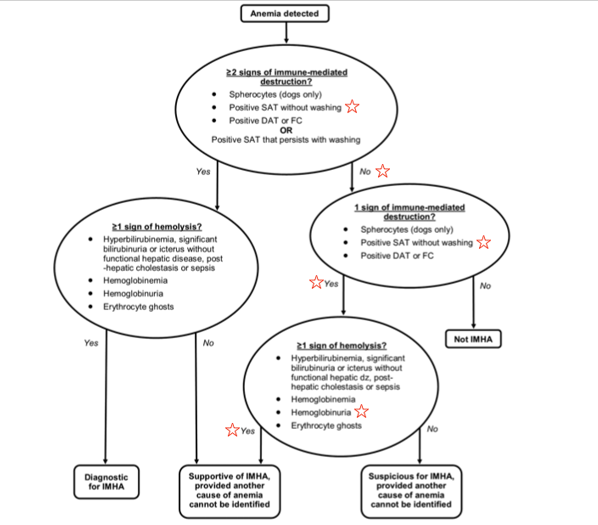
Figure 1: IMHA diagnostic algorithm as presented in the ACVIM consensus statement.
While an argument can be made for immune-mediated disease, when incorporating physical exam findings into the assessment of the patient’s anemia, decreased production or chronic anemia could be considered the most likely or substantial etiology. On physical exam, the patient was alert and responsive, pale, cachectic (BCS 2/9, MCS 1/5), eupneic, and had a normal heart rate (~100 BPM). These findings suggest chronic disease. In acute marked anemia (as seen with IMHA), tachypnea, tachycardia, and mentation changes or weakness would be expected. An elevated bilirubin would also be suspected given the degree of anemia.
RBC loss or hemorrhage was considered less likely and largely ruled out, as the ascites on necropsy was grossly serous fluid with no evidence of hematochezia, melena, and only mild hematuria (7 RBC/hpf). Ultimately, the cause of anemia in this patient is likely multifactorial.
Primary Splenic Torsion (PST):
Primary splenic torsion (PST) is rare and poorly understood. Acute and chronic PST have been reported, with English Bulldogs over-represented (11.8% of cases). Clinical signs of acute PST include abdominal pain and distention, vomiting, depression, and anorexia. Chronic PST presents with varying signs, including anorexia, weight loss, intermittent vomiting, abdominal distention, PU-PD, hemoglobinuria, and abdominal pain. It is difficult to determine the chronicity of the PST in this case.
The owner reported a few days of hyporexia, lethargy, and hematuria. However, the patient’s poor body and muscle condition suggests morbidities predating this timeline. Chronic gastric foreign body and enteropathy (presumed PLE) may have contributed to an acute onset of PST. English Bulldogs may have a genetic predisposition due to absent or weak gastrosplenic and/or phrenicosplenic ligaments.
Protein-Losing Enteropathy (PLE):
The lack of reported vomiting and diarrhea makes assumptions about chronic foreign body and PLE difficult. While diarrhea is the most commonly reported sign of PLE, up to 33% of cases have no diarrhea. The marked hypoalbuminemia and mucosal changes suggest PLE as the primary cause of hypoalbuminemia. GI biopsies would be necessary for definitive diagnosis.
Alternative etiologies include liver dysfunction, protein-losing nephropathies (PLN), malnutrition, and parasitism. While the liver appeared subjectively decreased in size, baseline lab work did not indicate liver dysfunction. A urine protein:creatinine ratio would help assess potential PLN following resolution of the urinary tract infection. Fecal analysis and empirical deworming are also indicated. However, given the degree of weight loss, hypoalbuminemia, and mucosal changes, chronic PLE remains the leading differential. Additionally, foreign body ingestion (pica) could be a manifestation of chronic GI disease in dogs.
Overall, this case highlights the value of ultrasound in identifying a complex constellation of clinical signs and pathologies in veterinary practice.
References:
- Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immune-mediated hemolytic anemia in dogs and cats. J Vet Intern Med. 2019;33(2):313-334. doi:10.1111/jvim.15441.
- Nelson RW, Couto CG. “Lymphadenopathy and Splenomegaly.” Small Animal Internal Medicine. Elsevier/Mosby, 2014.
- DeGroot W, Giuffrida MA, Rubin J, et al. Primary splenic torsion in dogs: 102 cases (1992-2014). J Am Vet Med Assoc. 2016;248(6):661-8. doi:10.2460/javma.248.6.661.
- “Pattern Changes.” eClinpath. 18 Oct. 2016. eclinpath.com.
- Thompson MS. Small Animal Medical Differential Diagnosis (Third Edition). W B Saunders Company, 2018.
- Simmerson SM, Armstrong PJ, Wünschmann A, et al. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J Vet Intern Med. 2014;28(2):331-7. doi:10.1111/jvim.12291.


